May 22, 2019 titusville, nj: janssen pharmaceutical companies; 2019. fda website: manufacturer. name of medicine [package insert]. u. s. food and drug. In the ensuing decades, the labeling information became the package insert (pis) or labeling content. pharmaceutical manufacturing companies evolved to. Antabuse medication comes in several medical forms: film-coated tablets, effervescent tablets, and dispersible tablets. the preparation medication information package insert is available in the following dosage strengths: 200 mg, 400 mg, and 500 mg. the drug should be taken daily, preferably at the same time in the morning. Highlights of prescribing information these highlights do not include all the information needed to use mircera safely and effectively. see. full prescribing information for mircera (methoxy polyethylene glycol-epoetin beta) injection, for intravenous or subcutaneous use initial u. s. approval: 2007. warning: esas increase the risk of death,.
How Do I Cite A Drug Package Insert Ask The Research Medical
A package insert is a document included in the package of a medication that provides information about that drug and its use. for prescription medications, the insert is technical, providing information for medical professionals about how to prescribe the drug. package inserts for prescription drugs often include a separate document called a "patient package insert" with information written in. 20223. See 17 for patient counseling information and medication guide. revised: 02/2021 warning: cytokine release syndrome and neurologic toxicities see full prescribing information for complete boxed warning. cytokine release syndrome (crs), including life-threatening reactions, occurred in patients receiving tecartus.
Apr 30, 2020 the package insert is a good source of information to use in addition to instructions your health care provider, nurse, or pharmacist may have given you. it is a good idea to review the package insert for any drug that you are newly taking, and to look at it again if anything about your health changes. . . . . . . . .

25119 . 26120 medication information package insert . 27123 . 30133 . 136 . .
()21 . Supplemental product information. information for dosing dogs. this insert describes the concurrent use of program (lufenuron) flavor tabs and capstar (nitenpyram)tablets for the management of fleas on dogs and puppies. please read the insert contained in each package for complete information on the individual products prior to dosing.
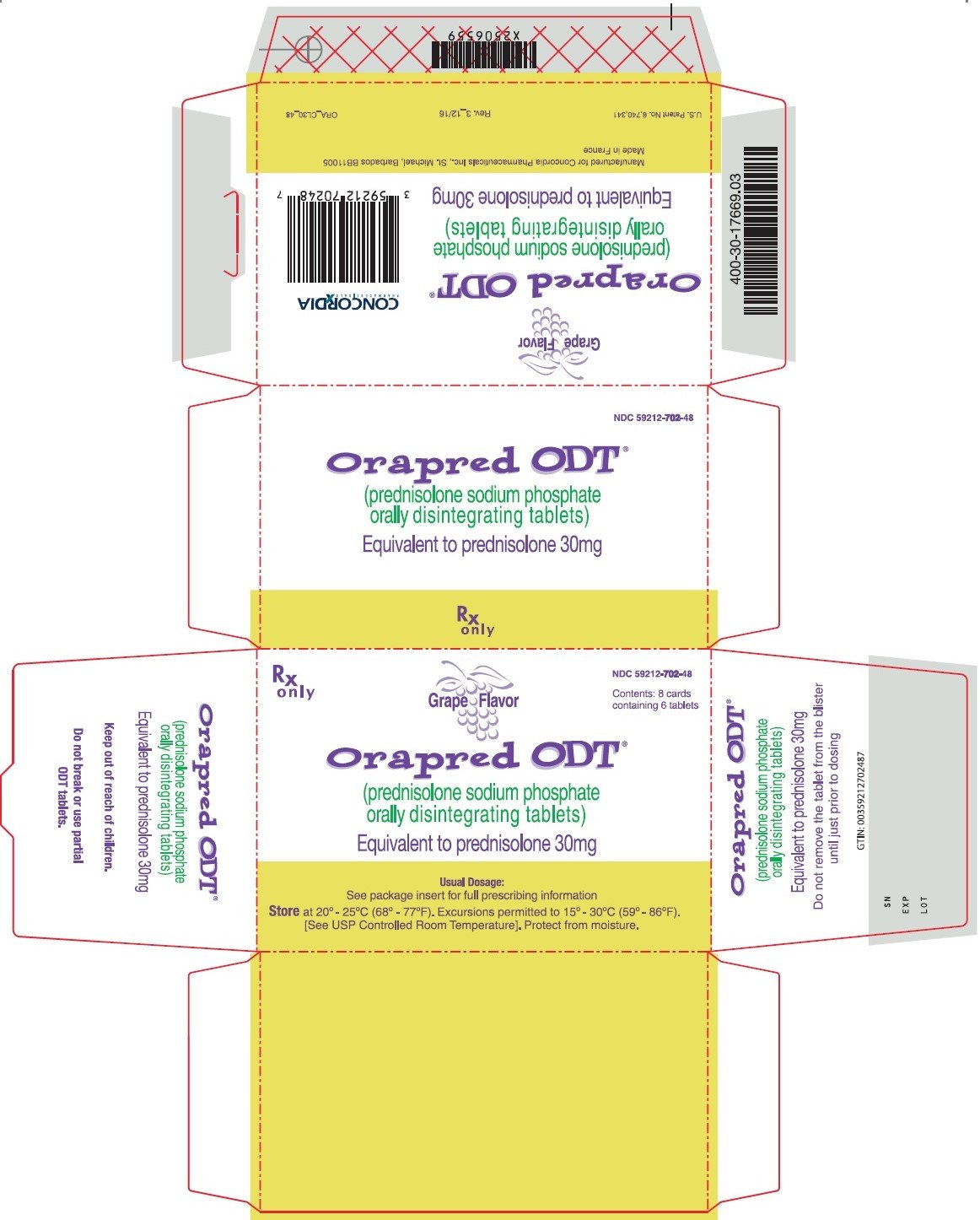
The purpose of the package insert (also known as prescription drug product insert or professional labeling) is to provide detailed drug information compiled and.
u 395-0001 603-1 tel 0265-52-2570 fax 0265-24-1978 . yoshikawa foundation all rights reserved tel. 0265-21-1981 395-0042 25 medication information package insert mail:info@yoshikawafoundation. or. jp. See 17 for patient counseling information and medication guide 05/2019 _____ full prescribing information: contents* warning: increased mortality in elderly patients with dementia-related psychosis; and suicidal thoughts and behaviors 1 indications and usage 2 dosage and administration 2. 1 general dosing information 2. 2 schizophrenia. The food and drug administration (fda or the agency) is proposing to.
: 3950824: : 47758: : : : 0265224750. Abstract: drug information on labels and inserts is a major source of knowledge for patients as they attempt to balance the risks and benefits of drugs and.
medication information package insert . . . . . . . . . . ,. Prescribing information. according to the fda, the approved prescribing information also called the package insert is the actual prescription drug label. the.

(:) e-shops . May 15, 2015 the intent of the pi is to create a learned intermediary between the manufacturer and the drugs user. although the learned intermediary is. Aug 31, 2006 otherwise, the only piece of information medication information package insert that must accompany each container of a drug product when it leaves the manufacturing facility is a pi.


0 komentar:
Posting Komentar